How To Determine If A Lewis Structure Is Polar Or Nonpolar
The hybridization number of CF4 is Sp and the steric number is 4. From the Lewis dot structure it is clear that the molecule is polar as it will have some net dipole moment along with the F-C-Cl bond.

How To Tell If A Bond Is Polar Or Nonpolar The Super Easy Way Youtube
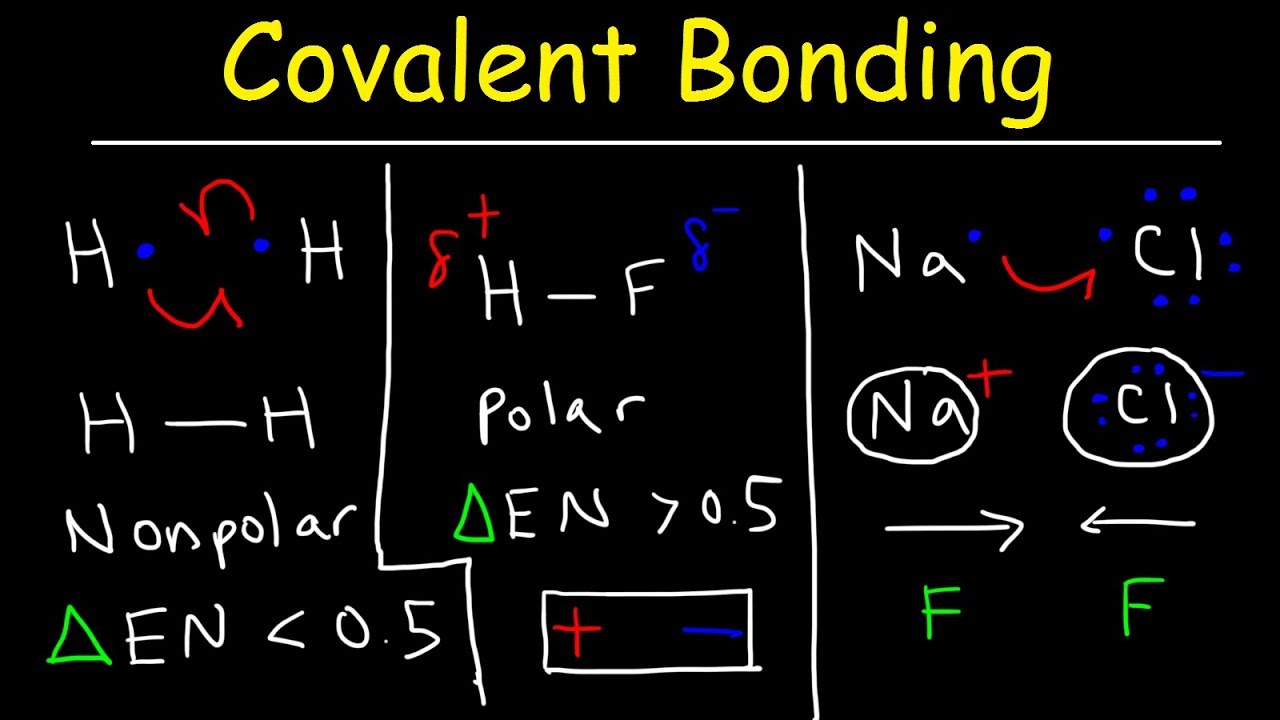
A covalent chemical compound is formed by bringing together multiple different.
How to determine if a lewis structure is polar or nonpolar. This chemistry video tutorial provides a basic introduction into polar and nonpolar molecules. CF4 is nonpolar in nature but the bond present in it is polar. The bond angle of CF4 is 1095.
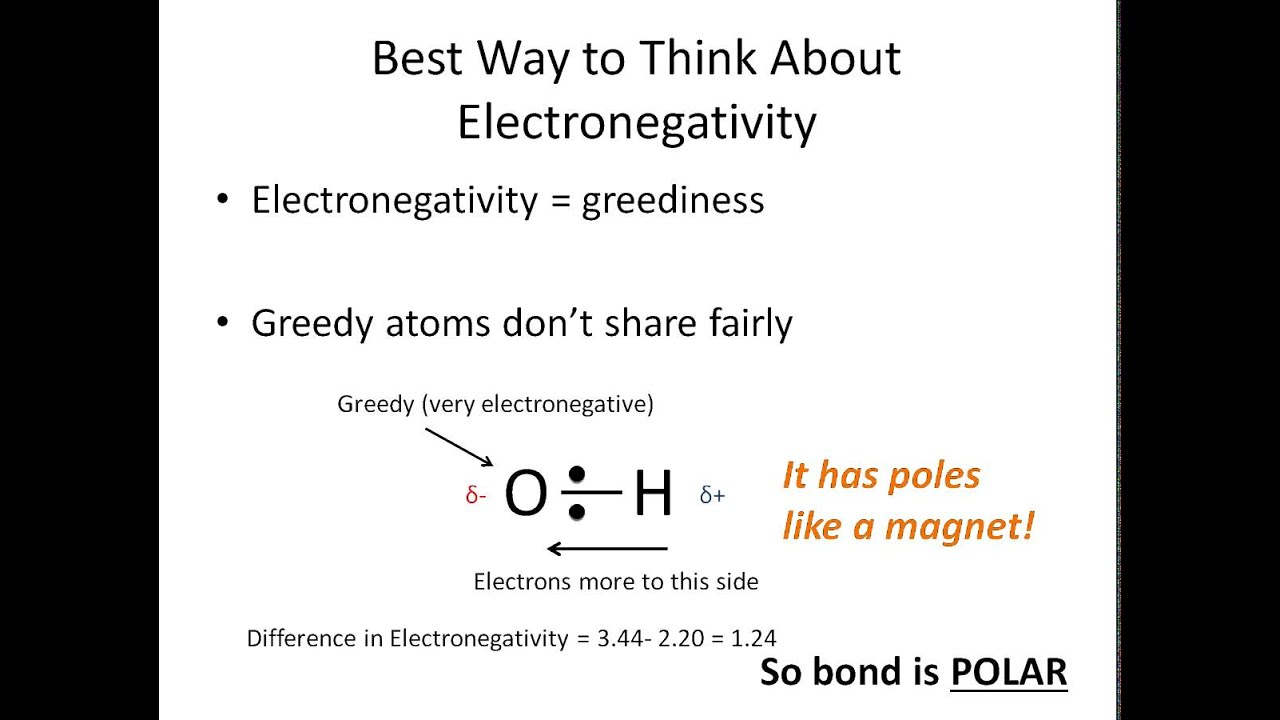
Examine the arrows in the Lewis dot structure. Higher the difference of electronegativity between the two different atoms more is the strength of polarity between them. Molecule Lewis structure Molecular Geometry is molecule polar Name all the intermolecular forces betwe around central or nonpolar.
The chemical and physical properties of any molecule can be determined if we know the Lewis Structure Molecular Geometry and Polarity of the molecule. In the Lewis structure the central Si atom has four electron groups with no lone pairs. It is used in many industries and more widely used in ethylene production.
If the arrangement is asymmetrical the molecule is polar. The total valence electron available for the CF4 lewis structure is 32. Notice that a tetrahedral molecule such as CCl 4 is nonpolar Figure 412.
If it is 20 or higher then the bond is considered an ionic bond. If you look at pictures of polar and nonpolar molecules they vary in symmetry. Also the electronegativity difference between the atoms plays a huge role to predict whether a molecule is polar or non-polar.
POSSIBLE ANSWERS TO THE SELF-CHECK EXERCISES Self-Check 1. Decide whether the arrangement of arrows is symmetrical or asymmetrical If the arrangement is symmetrical and the arrows are of equal length the molecule is nonpolar. To determine if a molecule is polar or nonpolar it is frequently useful to look at Lewis structures.
If the arrows are of different lengths and if they do not balance each other the molecule is polar. T h e B i g g e r E N T h e S m a l l e r E N If the resulting value is less than 04 then the bond is nonpolar covalent. If it is between 04 and 20 then the bond is polar covalent.
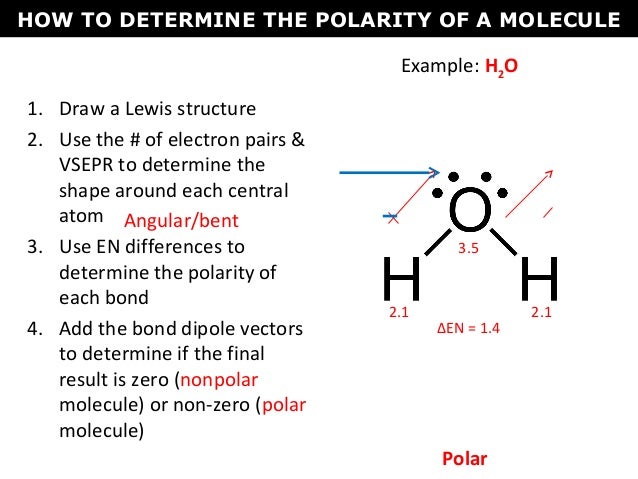
The Lewis structure will help you analyze the shape of the molecule given to you. To determine whether or not it is polar you must draw the Lewis structure and then determine the molecular geometry. How to Determine if a Molecule is Polar Or Nonpolar.
Then you take the difference or simply do. Determine which of the five categories of shapes your molecule falls into linear tetrahedral trigonal. These molecules atoms HCN CH Cs in middle HF H2O2 Os in middle CCIH HCO C in middle Analysis Questions.
The short and sweet answer is that if the ligands are the same then it is nonpolar. The electronegativity of bromine is 296 and for fluorine it is 398. Draw the 3D molecular structure w VSEPR rules Step 3.
Draw the Lewis structure Step 2. Click on the molecules name to see the answer but first try to do it yourself. Use symmetry to determine if the molecule is polar or non-polar.
Nonpolar compounds will be symmetric meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. This rule applies to all molecules except hydrocarbons and molecules with two atoms of the same element. Is C2H6 Polar or Non-polar.
Having a chemical formula of C2H6 it is made up of two Carbon and six Hydrogen atoms. Students should be able to look at the structures that are drawn and determine whether they are polar unequal charge distribution around the central atom or nonpolar equal charge distribution around the central atom. Therefore the type of IMF can be predicted.
Polar molecules have an unshared electron pair while the nonpolar molecule doesnt. Draw the Lewis structure for eqNH_2Cl eq and determine whether it is polar or nonpolar. The arrows are equal in length and the arrangement is symmetrical.
Start by drawing its Lewis structure. If the molecular geometry allows the polar bonds to cancel out such as in the linear geometry of CO 2 or the tetrahedral geometry of CH 4 then the molecule is nonpolar. Draw the Lewis structure and determine the polarity and intermolecular forces present in each molecule.
Write ionic Lewis structures for each including the covalent structure for the ion in brackets.
Polar Covalent Bonds And Nonpolar Covalent Bonds Ionic Bonding Types Of Chemical Bonds Youtube

Is Clf3 Polar Or Non Polar Clutch Prep

How To Determine If Molecule Is Polar Or Nonpolar Practice Problems Rules Examples Summary Youtube

Polar And Nonpolar Molecules How To Tell If A Molecule Is Polar Or Nonpolar Youtube

Polar And Nonpolar Molecules Youtube

Polar And Nonpolar Molecules Youtube

How To Tell If A Bond Is Polar Or Nonpolar The Super Easy Way Youtube
How To Determine If A Molecule Is Polar Or Non Polar Check Now
Post a Comment for "How To Determine If A Lewis Structure Is Polar Or Nonpolar"